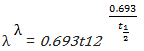
1. Waves
1.1. Progressive waves
a. describe what is meant by wave motion as illustrated by vibration in ropes, springs and ripple tanks.
b. understand and use the terms displacement, amplitude, phase difference, period, frequency, wavelength, and speed.
c. deduce, from the definitions of speed, frequency and wavelength, the wave equation v = f λ
d. recall and use the equation v = f λ
e. understand that energy is transferred by a progressive wave.
f. recall and use the relationship intensity ∝ (amplitude)2
1.2. Doppler effect
a. define Doppler effect.
b. understand that whenever there is a relative motion between the source of wave and the observer there is a change in the observed frequency.
c.
use the expression f0 = ![]() to
solve problems for the observed frequency for various cases: 1. moving source of
wave 2. moving observer 3. both moving source of wave and moving observer
to
solve problems for the observed frequency for various cases: 1. moving source of
wave 2. moving observer 3. both moving source of wave and moving observer
d. appreciate that Doppler effect is observed with all waves, including sound and light. (Applications of Doppler effect need not to be discussed in light)
2. Superposition
2.1. Interference, two source interference
a. state the principle of superposition of waves.
b. understand the terms interference, fringes, crest, trough and coherence.
c. explain the constructive and destructive interferences.
d. Discuss the conditions of constructive and destructive interference quantitatively.
e. show an understanding of experiments that demonstrate two source interference using water ripples.
f. show an understanding of two source interference in sound waves and light.
g. understand the conditions required if two-source interference fringes are to be observed.
h. use Young's double-slit experiment to calculate the wavelength of light.
i. recall and solve problems using the equation λ =
2.2. Diffraction
a. explain the meaning of the term diffraction.
b. show an understanding of experiments that demonstrate diffraction with both a wide gap and a narrow gap using a ripple tank. (you can use video or simulation to change both width gap and wavelength.
2.3 Diffraction Grating
a. define the diffraction grating.
b. recall and solve problems using the formula d sin θ = nλ
c. explain the production of the spectrum of white light with a diffraction grating.
2.4 Stationary waves in stretched strings
a. explain the formation of the stationary waves using the principle of superposition.
b. explain the terms node and antinode .
c. explain the formation of a stationary wave using a graphical method.
d.
derive and use the general expression for the frequency of the nth mode![]() =
=
e. calculate the speed of stationary waves in a stretched string.
3. Quantum physics
3.1 Energy of a photon
a. appreciate the particulate nature of electromagnetic radiation.
b. define the term photon. c) recall and use E = hf. (both in Joule and eV
3.2 Photoelectric emission of electrons
a. define photoelectric effect, threshold frequency and work function.
b. understand that the photoelectric effect provides evidence for a particulate nature of electromagnetic radiation while phenomena such as interference and diffraction provide evidence for a wave nature.
c. recall the significance of threshold frequency and use f=hfo
d. explain photoelectric phenomena in terms of photon energy and work function energy.
e. explain why the maximum photoelectric energy is independent of intensity, whereas the photoelectric current is proportional to intensity.
f. recall, use and explain the significance of hf=f+½ mv2max
g. describe an experiment to measure the maximum kinetic energy of photoelectrons.
h. define the stopping potential and use the concept of the loss of kinetic energy = the gain of electrical potential energy equation ½ mv2 max=eVo
i. study the graph of the maximum kinetic energy of photoelectrons against frequency or wavelength.
j. study the graph of the stopping voltage against frequency or wavelength.
3.3 Wave particle duality
a. describe and interpret qualitatively the evidence provided by electron diffraction for the wave nature of particles.
b. recall and use the relation for the de Broglie wavelength λ =
3.4 Energy levels in atoms and line spectra
a. show an understanding of the existence of discrete electron energy levels in isolated atoms (e.g. atomic hydrogen) and deduce how this leads to spectral lines.
b. distinguish between emission and absorption line spectra
c. recall and solve problems using the relation
4. Particle and nuclear pysics
4.1 Atoms, nuclei and radiation
a. infer from the results of the α-particle scattering experiment the existence and small size of the nucleus.
b. describe a simple model for the nuclear atom to include protons, neutrons and orbital electrons.
c. distinguish between nucleon number and proton number.
d. understand that an element can exist in various isotopic forms, each with a different number of neutrons.
e. use the usual notation for the representation of nuclides.
f. appreciate that nucleon number, proton number, and massenergy are all conserved in nuclear processes.
g. show an understanding of the nature and properties of α-, βand γ-radiations (both β– and β+ are included).
h. state that antineutrinos and neutrinos are produced during β– and β+ decay.
i. explain the changes occurs in a nuclide during a nuclear emission.
4.2 Mass defect and nuclear binding energy
a. show an appreciation of the association between energy and mass as represented by E = mc2 and recall and use this relationship.
b. understand the significance of the terms mass defect and mass excess in nuclear reactions.
c.
represent simple nuclear reactions by nuclear equations of the form
![]()
d. define and calculate mass defect, binding energy and binding energy per nucleon.
e. sketch the variation of binding energy per nucleon with nucleon number.
f. explain what is meant by nuclear fusion and nuclear fission with giving examples .
g. explain the relevance of binding energy per nucleon to nuclear fusion and to nuclear fission.
4.3 Radioactive decay
a. infer the random nature of radioactive decay from the fluctuations in count rate.
b. show an appreciation of the spontaneous and random nature of nuclear decay.
c.
define the terms activity and decay constant and recall and solve
problems using
![]()
d. infer and sketch the exponential nature of radioactive decay and solve problems using the relationship x = x0 where x could represent activity A, number of undecayed nuclei N or received count rate R.
e. define half-life.
f. solve problems using the relation