Unit 7 – Introduction to Organic Chemistry
7.1 Functional Groups and their Formulae
Functional Groups of Organic Compounds
-
Functional groups determine the physical and chemical properties of molecules
-
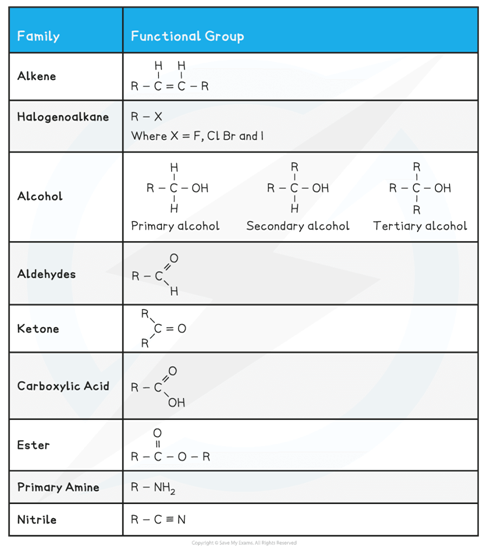
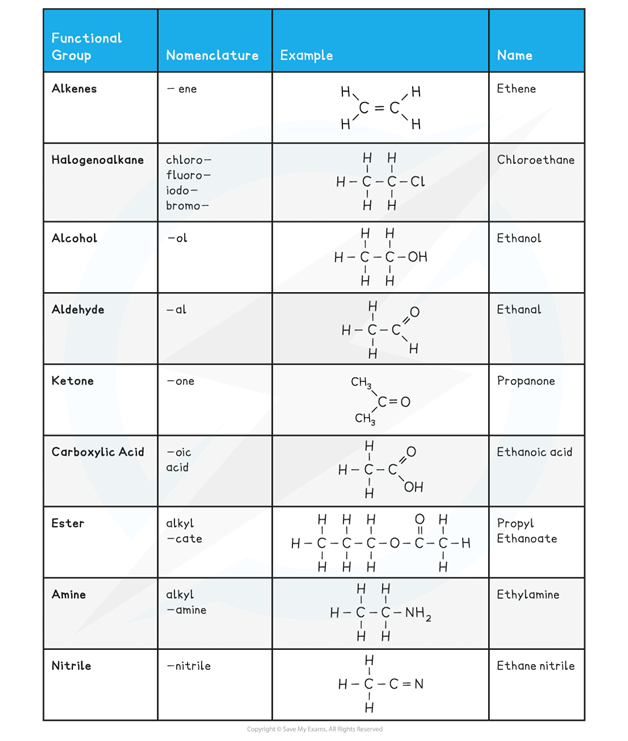
The table below shows a summary of common functional groups found in compounds
-
R is any other atom or group of atoms (except for hydrogen)
Functional groups found in compounds table
Formulae of Organic Compounds
-
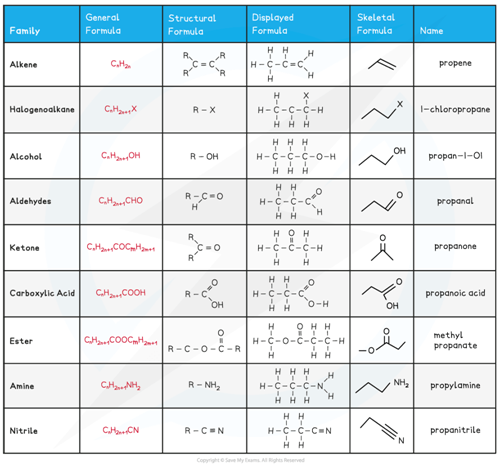
The general formula is a formula that represents a homologous series of compounds using letters and numbers
-
Eg. the general formula of alkanes is CnH2n+2
-
A homologous series is a group of organic compounds that have the same functional group, the same general formula and the same chemical properties
-
-
The structural formula is a formula that shows how the atoms are bonded to each carbon atom in a molecule
-
The displayed formula is a 2D representation of an organic molecule showing all its atoms (by their symbols) and their bonds (by single, double or triple bonds)
-
The skeletal formula is a simplified displayed formula with all the carbon and hydrogen (C-H) bonds removed
Overview of the formulae of organic compounds table
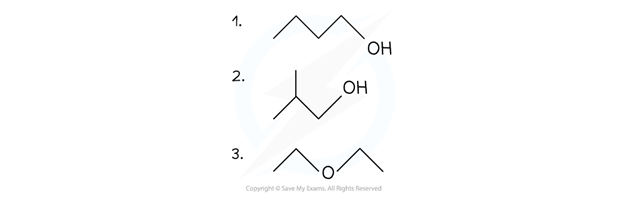
Worked example: Drawing skeletal formulae of molecules

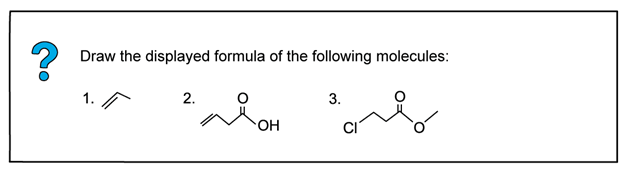
Worked example: Drawing displayed formulae of molecules
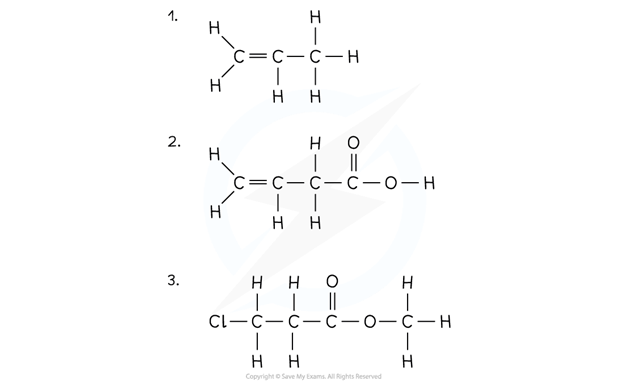
3.1.3 Naming of Organic Compounds
Nomenclature of Aliphatic Compounds
-
Systematic nomenclature can be used to name organic compounds and therefore make it easier to refer to them
-
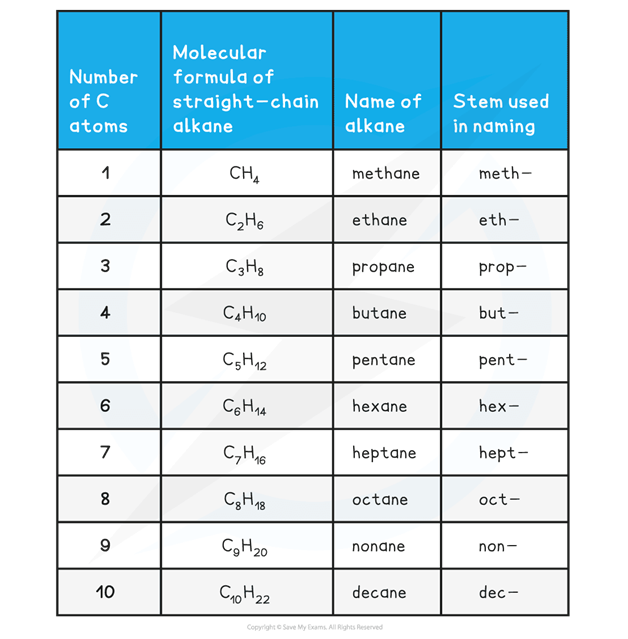
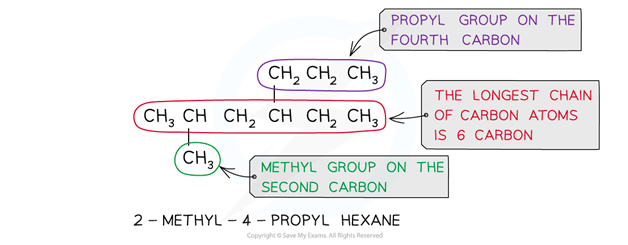
The alkanes provide the basis of the naming system and the stem of each name indicates how many carbon atoms are in the longest chain in one molecule of the compound
Nomenclature of organic compounds table
-
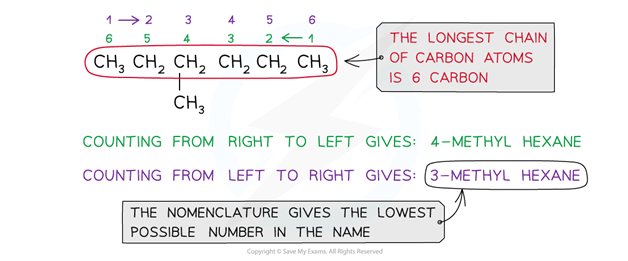
If there are any side-chains or functional groups present, then the position of these groups are indicated by numbering the carbon atoms in the longest chain starting at the end that gives the lowest possible numbers in the name
-
The hydrocarbon side-chain is shown in brackets in the structural formula
-
CH3CH(CH3)CH2CH3
-
The side-chain is named by adding ‘-yl’ to the normal alkane stem
-
This type of group is called an alkyl group
-
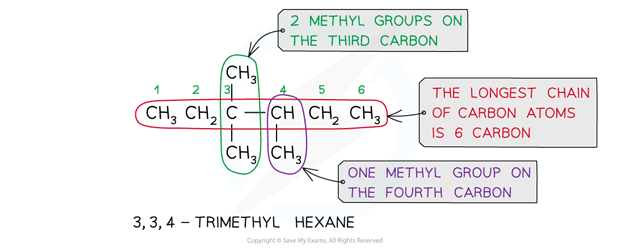
If there are more than one of the same alkyl side-chain or functional groups, di- (for two), tri- (for three) or tetra- (for four) is added in front of its name
-
The adjacent numbers have a comma between them
-
Numbers are separated from words by a hyphen
-
If there is more than one type of alkyl side-chain, they are listed in alphabetic order
Functional groups & their nomenclature table
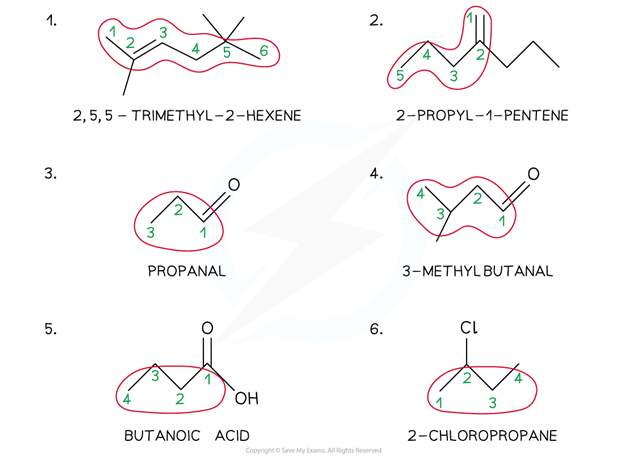
Worked example: Naming organic molecules
7.2 Isomerism
Structural Isomerism
Structural Isomerism: Chain, Position & Functional Group
-
Structural isomers are compounds that have the same molecular formula but different structural formulae
-
Eg. propene and cyclopropane
-
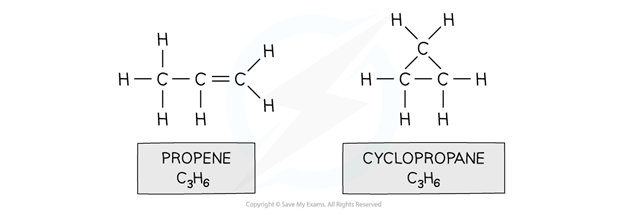
Both propene and cyclopropane are made up of 3 carbon and 6 hydrogen atoms but the structure of the two molecules differs
-
There are three different types of structural isomerism:
-
Chain isomerism
-
Positional isomerism
-
Functional group isomerism
-
Chain isomerism
-
Chain isomerism is when compounds have the same molecular formula, but their longest hydrocarbon chain is not the same
-
This is caused by branching
-
Eg. pentane and 2,2-dimethylpropane
-
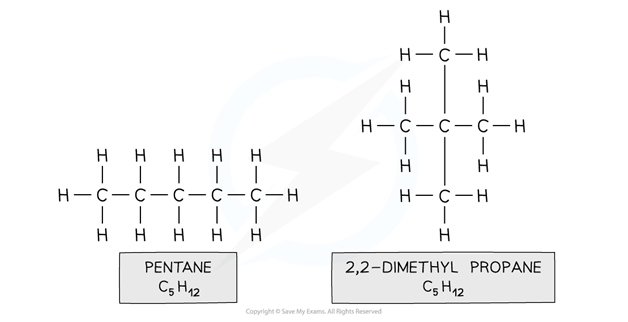
Both compounds are made up of the same atoms however the longest carbon chain in pentane is 5 and in 2,2-dimethylpropane 3 (with two methyl branches)
Positional isomerism
-
Positional isomers arise from differences in the position of a functional group in each isomer
-
The functional group can be located on different carbons
-
For example, butanol and 2-butanol
-
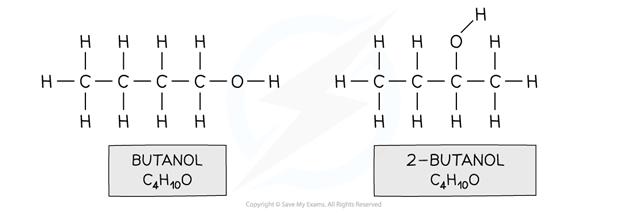
Both compounds have an alcohol group and are made up of 4 carbon, 10 hydrogen and one oxygen atom however in butanol the functional group is located on the first carbon and in 2-butanol on the second carbon
Functional group isomerism
-
When different functional groups result in the same molecular formula, functional group isomers arise
-
The isomers have very different chemical properties as they have different functional groups
-
For example, butanol and ethoxyethane
-
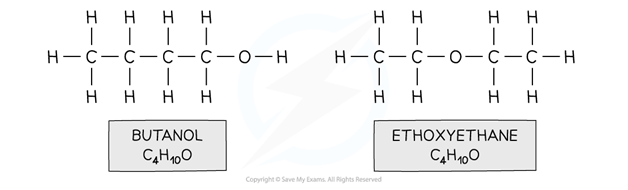
Stereoisomerism
Stereoisomerism: Geometrical & Optical
-
Stereoisomers are compounds that have the same atoms connected to each other, however the atoms are differently arranged in space
-
There are two types of stereoisomerism:
-
Geometrical (cis/trans) isomerism
-
Optical isomerism
-
Geometrical (cis/rans) isomerism
-
Geometrical isomerism is seen in unsaturated (double bond containing) or ring compounds that have the same molecular formula and order of atoms (the atoms are connected similarly to each other) but different shapes
-
Cis/trans nomenclature is used to distinguish between the isomers
-
Cis isomers have functional groups on the same side of the double bond/carbon ring
-
Trans isomers have functional groups on opposite sides of the double bond/carbon ring
-
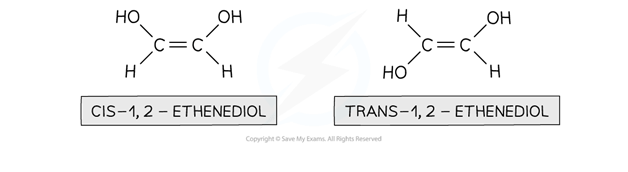
Geometrical isomerism in unsaturated compounds
