1. Atom is the smallest particle of a substance that can not be broken further.
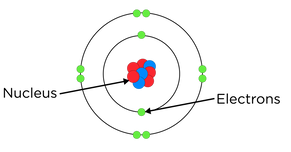
2. Atoms are made up of three types of sub-atomic particles – protons, neutrons, and electrons
3. Protons and neutrons make up the nucleus, where most of the mass is concentrated.
Electrons orbit the nucleus in shells
|
Particle |
Relative Mass |
Relative Charge |
|
Proton |
1 |
+1 |
|
Neutron |
1 |
0 |
|
Electron |
1/1840 |
-1 |
Mass Number
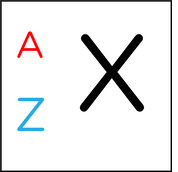
- Element, X
- Mass number, A, is the total number of protons and neutrons in the nucleus.
- Atomic number, Z, The number of protons in the nucleus of an atom
- The number of positively charged protons is equal to the number of negatively charged electrons in an atom, making the atom neutrally charged
- Mass number = number of protons + number of neutrons
- Atomic number = number of protons = number of electrons
Atoms and Periodic table
Elements are arranged in order of atomic (proton) number (bottom number) and so that elements with similar properties are in columns, known as groups.
● Elements in the same periodic group have the same amount of electrons in their outer shell, which gives them similar chemical properties.
● Elements are arranged in order of increasing atomic number, in rows called periods and elements, with similar properties are placed in the same vertical columns called groups.
-------------------------------------------------------------------------------------------------------------
Electron arrangement
1. Electrons are arranged around the nucleus in shells.
2. Starting with the first shell (closest to the nucleus), each shell is filled with electrons before any further shells gain any electrons
* First shell can have up to 2 electrons
* Second shell can have up to 8 electrons
* Third shell can have up to 8 electrons
When reacting, all atoms will try to acquire this perfect arrangement of electrons – i.e. having the maximum number of electrons as possible in their outer shell – therefore, all atoms try to have 8 electrons in their outer shell (unless they only have one shell then they will try to have only 2) because this is the most stable arrangement
● Nobles gases have 8 electrons in their outer shells already making them very stable so unreactive
Homework .
Draw the electron structure for the atoms with proton number :
a) 16 b)20 c) 17
-----------------------------------------------------Isotopes
Isotopes are the atoms of the same element which have the same proton number but a different nucleon number (different number of neutrons).
Isotopes have the same chemical properties because they have the same number of electrons in their outer shell and the number of electrons in the outer shell is responsible for chemical reactions
Example: Isotopes of carbon are :
Carbon – 12
Carbon- 13
Carbon – 14
Two types of isotopes are
(a) Radioactiveisotopes : Isotopes those emit radiations
(b)Non-radioactiveisotopes : Isotopes those do not emit radiations.
Uses of radioactive isotopes ;
Medical uses:
1. Sterilisingsurgical equipment
2. Cancer treatment in radiotheraphy
Industrial uses of radioactive isotopes
1. Radioactive isotopes are used in Smoke alarms
2. They are used in detecting leakage in gas pipes
https://youtu.be/UYvx0O8itMA- Isotopes
