Grade 9 Chemistry
Notes
Unit 1: The particulate nature of matter
1.State the distinguishing properties of solids, liquids and gases
The three states of matter are solid, liquid and gas
● Melting and freezing take place at the melting point
● Boiling and condensing take place at the boiling point
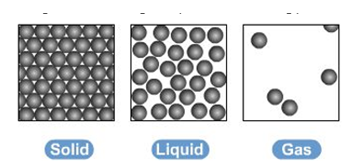
● They can be represented by the simple model above, particles are represented by small solid spheres
● solids- particles have a regular arrangement and are close together
● liquids- particles have a random arrangement and are close together
● gases- particles have a random arrangement and are spread apart
2.Describe the structure of solids, liquids and gases in terms of particle separation, arrangement and types of motion.
· Gas: particles have the most energy – shown by the diagram, as the particles are the most spread apart, motion is more random and frequent
● Liquid: particles have more energy than those in a solid, but less than those in a gas
● Solid has least energy – particles are not moving/are just vibrating
3.Describe changes of state in terms of melting, boiling, evaporation, freezing, condensation and sublimation
Physical changes – therefore involves the forces between the particles of the substances, instead of these changes of state being chemical changes
Evaporation = happens at the surface, molecules have enough energy to evaporate ( start to change Liquid to gas)
Freezing = liquid to solid
Melting = solid to liquid
Boiling = happens throughout the liquid, liquid to gas ( Formation of bubbles)
Condensation = gas to liquid
Sublimation = solid to gas
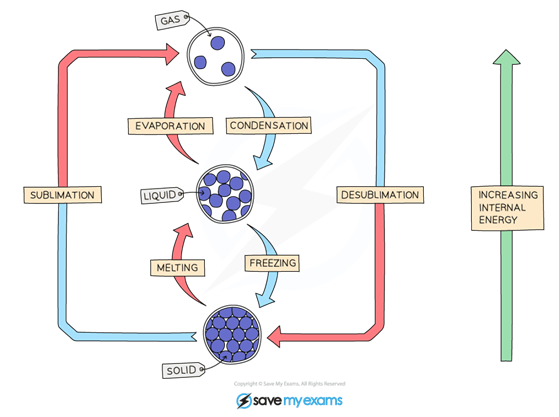
4.Explain changes of state in terms of the kinetic theory.
Kinetic theory can help to explain melting, boiling, freezing and condensing…
o The amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles of the substance.
o The nature of the particles involved depends on the type of bonding and the structure of the substance.
o The stronger the forces between the particles the higher the melting point and boiling point of the substance.
o The more kinetic energy (from increased temperature) particles have, the more movement, which causes a change of state from (s) to (l) to (g)
5. Describe qualitatively the pressure and temperature of a gas in terms of the motion of its particles.
The higher the pressure = the more motion of a gas’ particles
● The higher the temperature = the more motion of a gas’ particles
6. Show an understanding of the random motion of particles in a suspension (sometimes known as Brownian motion) as evidence for the kinetic particle. (atoms, molecules or ions) model of matter
Particles in liquids and gases (known as fluids) move randomly (this is called Brownian motion) ● This happens because they collide with other moving particles in the fluid ● This is evidence for the kinetic particle model of matter- it shows that there are individual particles which make up solids/liquids/gases
Particles in liquids and gases move randomly because they are bombarded by the other moving particles in the fluid. Larger particles can be moved by light, fast-moving molecules
Robert Brown observed the random movement of pollen grains within water, which showed that there were separate particles within the water that were moving randomly and caused the grain to move (kinetic theory)
7.Explain diffusion.
Movement of particles from an area of high concentration to an area of low concentration ● For this to work, particles must be able to move. Therefore, diffusion does not occur in solids, since the particles cannot move from place to place (only vibrate).
A smell does not travel very fast, because the partices collide with particles of air, changing direction randomly when they collide, taking much longer to travel from place to place
8.Explain dependence of rate of diffusion on molecular mass.
The smaller the molecular mass, the greater the average speed of the molecules (but all gases have the same average kinetic energy at the same temperature) o Therefore, the smaller the molecular mass, the faster the gas diffuses.
